Abstract
Hydroxyurea (HU) is the only medication available with neuroprotective potential in sickle cell anemia (SCA), yet the physiological mechanisms of this effect remain unclear. We utilized MRI to measure cerebral blood flow (CBF) and oxygen extraction fraction (OEF) to test our hypothesis that HU therapy relieves ongoing cerebral metabolic stress by decreasing OEF in patients with SCA.
Brain MRI measured CBF (pseudo-continuous arterial spin labeling), OEF (asymmetric spin echo sequence that measures voxel-wise tissue deoxyhemoglobin) and vasculopathy (magnetic resonance angiography) in prospectively enrolled participants with SCA. FMRIB's Linear Image Registration Tool individually co-registered CBF and OEF maps to corresponding T1 images, and FMRIB's Automated Segmentation Tool segmented gray and white matter (GM and WM). For analysis, participants were cohorted by SCA therapy they were receiving at the time of the MRI. Group comparisons between SCA without HU, SCA with HU, and SCA pre-transfusion (pre-tx; MRI up to 24 hours before chronic tx therapy) were made with Kruskal-Wallis or chi-squared test, followed by pairwise comparisons with Mann-Whitney U test. Bivariate correlations were described with Spearman's rho. Significance was specified as p-value < 0.05, and the Benjamini-Hochberg procedure controlled for an overall family-wise error rate of 0.05.
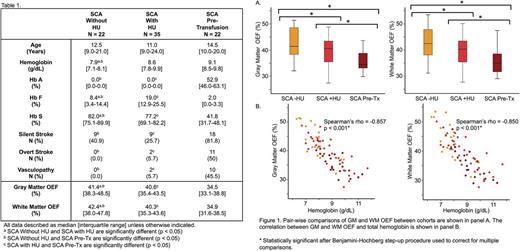
Seventy-nine participants with SCA (70 HbSS, 9 HbSβ0 thalassemia) underwent MRI. There was no significant difference in age (p = 0.965) or sex (p = 0.471) among treatment cohorts. Hemoglobin (Hb) was significantly different between cohorts (p = 0.001). While the SCA without HU cohort had a significantly lower Hb than both SCA with HU (p = 0.010) and SCA pre-tx (p < 0.001) cohorts, there was no significant difference in Hb between SCA with HU and SCA pre-tx (p = 0.201). As expected, the SCA with HU cohort had a higher % Hb F compared to the SCA without HU (p < 0.001) and SCA pre-tx (p < 0.001) cohorts, while the SCA without HU cohort had higher % Hb S than the SCA with HU (p = 0.048) and SCA pre-tx (p < 0.001, Table 1) cohorts. SCA participants received a median dose of 25.7 [20.1-31.1] mg/kg/day of HU. Table 1 shows the prevalence of stroke and vasculopathy in each cohort.
There was no difference in GM (p = 0.680) or WM (p = 0.736) CBF among the 3 cohorts, even after controlling for age with multiple regression. However, there was a significant difference in GM OEF among cohorts (p < 0.001), with significant pairwise differences between SCA without HU versus SCA with HU (p = 0.040), and SCA pre-tx versus SCA without HU (p < 0.001) and SCA with HU (p = 0.015, Table 1, Figure 1A). A similar difference in WM OEF was observed among the 3 cohorts (p = 0.001), with significant pairwise differences between SCA without HU versus SCA with HU (p = 0.049), and SCA pre-tx versus SCA without HU (p < 0.001) and SCA with HU (p = 0.010, Table 1, Figure 1A).
To investigate potential mechanisms of metabolic stress reduction, we evaluated the relationship between OEF, total Hb, % Hb A, % Hb F and % Hb S. OEF and Hb were strongly correlated in both GM and WM (GM: spearman's rho = -0.857, p < 0.001; WM: spearman's rho = -0.850, p < 0.001; Figure 1B), with weaker correlations seen between OEF and % Hb S (GM: spearman's rho = 0.365, p = 0.001; WM: spearman's rho = 0.372, p = 0.001) and % Hb A (GM: spearman's rho = -0.348, p = 0.002; WM: spearman's rho = -0.373, p = 0.001). WM OEF correlated with % Hb F (spearman's rho = 0.248, p = 0.033), but the correlation between GM OEF and % Hb F was not significant (p = 0.072). All significant univariate correlations were entered into a stepwise multiple linear regression of GM and WM OEF. Total Hb (β = -0.039, p < 0.001) and % Hb F (β = 0.001, p = 0.003) were independent predictors in the final model for GM OEF (R2 = 0.737, p = 0.003), and for WM OEF (total Hb: β = -0.041, p < 0.001; % Hb F: β = 0.001, p = 0.001; R2 = 0.719, p < 0.001). After exclusion of cohort, % Hb S and % Hb A, the final models were valid without collinearity.
We report the first investigation into the effects of HU on cerebral oxygen metabolism in SCA. Our data suggest that HU mitigates ongoing cerebral metabolic stress by improving cerebral oxygen delivery, allowing reduction in OEF without altering CBF; however, the degree of OEF correction is not as strong as chronic tx therapy. Longitudinal evaluation of these cohorts is needed to determine if the improvement in OEF by HU is sufficient for prevention of stroke and cognitive dysfunction in patients with SCA.
Fields: Proclara Biosciences: Equity Ownership. Hulbert: Pfizer: Other: Spouse employment at Pfizer.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal